Gene: MYO7A
Year Identified: 1995
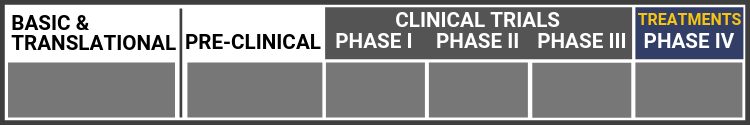
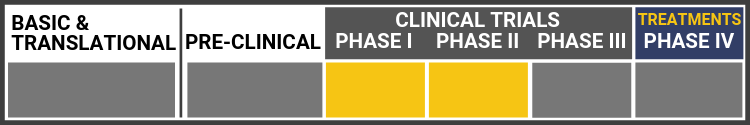
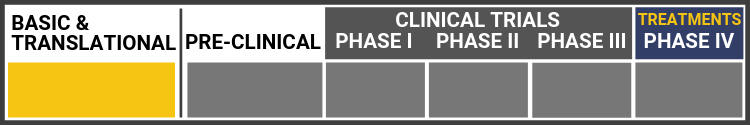
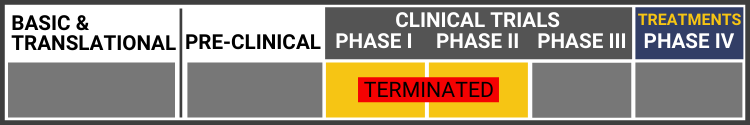
Each research project listed below will include a graphic of the research continuum. The gold box indicates where this project falls on the continuum, illustrating its progress towards reaching people living with Usher syndrome, from "Bench to Bedside."
Click here to learn more about the different stages in the research continuum.
USH1B-Related Science News
In late 2024, AAVantgarde shared exciting announcements on the first participant dosage, Orphan Drug Designation, and early safety data in the Phase 1/2 LUCE-1 clinical trial for vision loss caused by Usher syndrome type 1B.
To develop successful treatments, scientists must understand disease etiology and its corresponding biomarkers, which are biological molecules.
Italy’s Center for Rare Ocular Diseases at the University of Campania designed and conducted a longitudinal natural history study of European patients with confirmed Usher syndrome diagnosis and biallelic MYO7A variants.
Usher syndrome (USH) is classified into three major clinical subtypes (types 1–3), with type 1 being the most severe.
This study used a special type of 3D imaging to look at tiny parts of inner ear cells in zebrafish.
Martha Neuringer, Ph.D., leads the research team at OHSU, Oregon Health & Science University, that confirmed the first-ever nonhuman primate model of Usher syndrome.
Dr. Neuringer's lab created a monkey with the MYO7A mutation that causes Usher Type 1B. For the first time, an animal model demonstrates all three phenotypes of USH1B: deafness, impaired balance and retinal degeneration.
This is significant because primates are the closest genetic cousins to humans, and having this animal model allows scientists to better understand Usher syndrome and test potential treatments.
MYO7A is a large gene that encodes myosin VIIA, a protein that helps maintain stereocilia in the inner ear and the retinal pigment epithelium in the retina.
Researchers used CRISPR/Cas9 technology to disrupt the MYO7A gene in monkeys to create a nonhuman primate model for Usher syndrome type 1B (USH1B).
Usher syndrome is the most common cause of deafness associated with visual loss of a genetic origin. The purpose of this paper is to report very severe phenotypic features of type 1B Usher syndrome in a Saudi family affected by a positive homozygous splice site mutation in MYO7A gene. This mutation manifested with advanced retinal degeneration at a young age.
What this means for Usher syndrome: Individuals with this particular mutation may experience more severe symptoms than other Usher 1B patients.
In this USH Talk, Dr. Shannon Boye summarizes efforts to develop a dual AAV vector-based gene therapy for Myosin7a Usher syndrome (USH1B). The drawbacks of USH1B mouse models and a rationale for testing these vectors in a more clinically relevant species are discussed.
Jennifer Phillips, Ph.D." on defining “Failure”: Disclosing when things don’t work and understanding WHY is a really important, though often overlooked realm of research. Here are a couple of USH1 research stories from today’s presentations that illustrate that point.
This review, published by Williams et al discusses possible gene therapy approaches for the prevention of retinal degeneration in Usher syndrome.
João Carlos Ribeiro, Bárbara Oliveiros, Paulo Pereira, Natália António, Thomas Hummel, António Paiva & Eduardo D. Silva
Study aimed at identifying and characterizing putative differences in olfactory capacity between patients with USH and controls, as well as among the subtypes of USH.
Hidekane Yoshimura, Maiko Miyagawa, Kozo Kumakawa, Shin-ya Nishio, and Shin-ichi Usami.
This first report describing the frequency (1.3–2.2%) of USH1 among non-syndromic deaf children highlights the importance of comprehensive genetic testing for early disease diagnosis.
Gene therapy is still a relatively new development, and so far, the only USH target being delivered via viral vector in clinical trials is MYO7A (USH1B). There are a few different reasons for this, but all boil down to a numbers game.
Three patients have been treated so far with no serious adverse events after six months. They have been allowed to proceed to delivering a larger dose to the next group of patients.
The bulk of the presentations I took in today were reports from clinicians treating Usher patients. I don’t get to interact with clinicians on a regular basis, so it is hugely instructive for me to get their perspective on diagnosis and monitoring of the progressive retinal degeneration seen in Usher syndrome
Today was an 11-hour maelstrom* of really good science. Of all the great research stories I heard, there are several that will likely be of interest to our readers:
At last year’s ARVO conference there was a presentation reporting successful animal testing for a gene therapy product called “UshStat”*. While this work has not yet appeared in a peer-reviewed publication the ARVO abstract can be found here. The poster presentation at the meeting described the use of a non-pathogenic viral vector to deliver a normal copy of the gene affected in Usher Type 1B (MYO7A) into the retina.
In preparing to write this blog post, I planned to leap right into the meat of the study, because I know that’s what you all are most keen to hear about. However, once I got into it I realized that I’d be doing our readers a disservice by cutting to the juicy center without context.
The US Food and Drug Administration (FDA) has approved Oxford BioMedica's Investigational New Drug (IND) application for the Phase I/IIa clinical development of UshStat to treat Usher syndrome type 1B. Oxford Biometica will enroll 18 patients with Usher type 1b at the Casey Eye Institute in Portland, Oregon. The study will be lead by Dr. Richard Weleber.
This 2010 review dives into the genetics of pathological mechanisms of Usher syndrome.
What this means for Usher syndrome: Research has come a long way since 2010!
It has been discovered that a myosin protein connected to Usher syndrome works differently from many other myosins.