Are we there yet? A clinical trial to test a potential gene therapy for Usher 1B
May 3, 2012
by Jennifer Phillips, Ph.D.
In my last post, I summarized over a decade of research on the putative functions of the USH1B protein, MYO7A, in retinal cells. I did this in hopes that the forthcoming descriptions of the preclinical and clinical trials for this therapy would have a bit more relevance, so do let me know how that works out for you. Here goes:
At last year’s ARVO conference there was a presentation reporting successful animal testing for a gene therapy product called “UshStat”*. The poster presentation at the meeting described the use of a non-pathogenic viral vector to deliver a normal copy of the gene affected in Usher Type 1B (MYO7A) into the retina.
I’ve described the procedure for this type of viral delivery system previously, but briefly, these are virus strains that have been altered in a lab to disable whatever disease-causing effects they might have and to incorporate the genetic code for a functional copy of the gene of interest into the viral DNA. Once the virus has been modified to carry this ‘payload’, a solution is prepared containing many copies of this virus, which is then injected into the space behind the eye. Although the pathogenic nature of the virus has been altered, it still retains the ability of wild viruses to ‘dock’ with and insert viral DNA into an animal cell for the purpose of propagating itself. So, once injected adjacent to the retina, the virus enters the retinal cells and delivers the payload—a healthy version of a gene these cells require for normal function.
The researchers conducting the preclinical trial with UshStat used the shaker1 mouse I described in the last post, with the light-sensitive genetic background, to test the effects of this gene replacement therapy.
Recall that in these sensitized shaker1 mice, the myo7a mutation caused problems with transporting proteins within the photoreceptors. When mice are adapted to a dark environment, in which the fast-acting rod photoreceptors are primed to detect any tiny scrap of light that happens along, a protein called transducin inhabits the outer segment—the light receiving region—of the rods. When shifted to a more brightly lit environment, in which cone photoreceptors take on more of the light-sensing responsibility, this protein migrates down into lower regions of the photoreceptors. This light-dependent protein relocation can be seen in Panels A and B of the figure below:
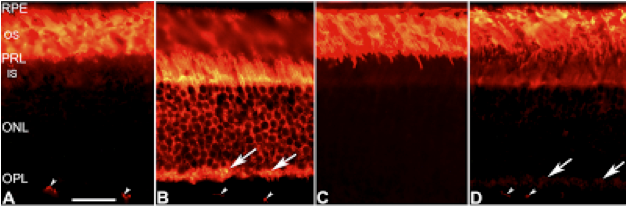
From Peng et al, 2011
Panel A shows transducin protein, labeled orange, in a normal, ‘wild-type’, retina from a mouse that was dark adapted before being sacrificed for this experiment. Panel B shows a retina from a wild-type mouse that was dark adapted for several hours, just like the mouse in panel A and then shifted to a lighter environment for 1 hour before its demise.
Panels C and D are taken from sensitized shaker1 mice treated to the same light conditions as their wild-type brethren in A and B. C shows the dark adapted shaker1 mouse retina, which looks pretty similar to the normal retina in panel A. But as you can see in panel D, the protein migration after 1 hour of light exposure is no where near as robust as what we saw in panel B from the wild-type mouse. The shaker1 just can’t move things as efficiently as it seems, and although there is no experiment so far that has shown a direct connection between failure to efficiently move transducin and the subsequent retinal degeneration exacerbated by bright light conditions in sensitized shaker1 mice, it certainly seems plausible that such damage could result from having an accumulation of a light-reactive molecule like transducin in the wrong place. The authors showed that factors other than transducin also accumulate inappropriately in the sensitized shaker1 mouse retinas, so cell death may result from accumulation of some other protein, or the combination of some or all of these ‘traffic-jams’.
So, enter the preclinical trials of UshStat. No pictures to show here, but the abstract linked to earlier in this piece describes two major results when the sensitized shaker1 mice were treated with this gene replacement therapy:
- Transducin relocation in response to bright light became more efficient
- Light induced photoreceptor degeneration was rescued.
Again, it’s not known whether restoration of proper trafficking of transducin in particular is saving the photoreceptors from death, or some other factor, but it is probably fair to think of transducin as a ‘reporter’ of efficient trafficking from the outer segment in these experiments. At any rate, it’s a powerful finding, and, probably along with other studies that haven’t been made public yet, sufficiently convincing to the review board who approved the clinical trials that are just getting underway.
Obviously we won’t be able to view protein trafficking in the eyes of the Ush1B patients undergoing the trial, but in time we should know whether the treatment is forestalling their progressive retinal degeneration and preserving their vision over what the typical Ush1B disease progression would be. I wish them all the very best, and thank them for volunteering for this milestone study. Watch this space for further updates on this trial as they become available.
*UshStat is a registered trademark but I don't know how to put the little R in a circle next to the name throughout the post. So just know it's a trademark when you see the name.
Reference: Peng, Y-W., Zallocchi, M., Wang W-M., Delimont, D and Cosgrove, D. (2011) Moderate light-induced degeneration of rod photoreceptors with delayed transducin translocation in shaker1 mice. IOVS 52 (9): 6421-6427.







