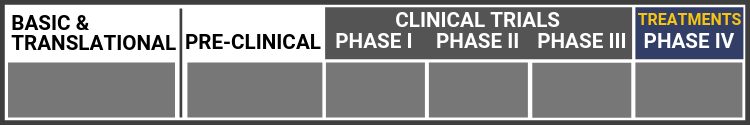
Research Continuum Definitions
-
Basic Research
An essential starting point to understand how a disease is caused or how it affects humans. This is done in labs, where scientists study the core building blocks of life - DNA, cells, proteins, molecules, etc. — to answer fundamental questions about their structures and how they work.
-
Translational Research
Acts as a bridge between science and practice. It refers to the application of basic scientific findings in a laboratory setting into potential treatments for disease. This research involves both scientists and clinicians who conduct their own research but exchange information with each other frequently. Translational research is needed to show that a treatment approach works in some living system before it is used on humans.
-
Preclinical Study
This is a study to test a drug, a procedure, or another medical treatment in animal models. In drug discovery, researchers may search through hundreds of millions of molecules in 'molecule libraries' to see if any 'match' or 'interact' with the disease target. The aim of a preclinical study is to collect data in support of the safety of the new treatment. Preclinical studies are required before clinical trials in humans can be started.
-
Clinical Trial Phase I
The aim of Phase I trials is to test the safety of the treatment in a small group, not whether the treatment works.
-
Clinical Trial Phase II
Phase II trials test the drug or treatment approach in a small group of patients to see if it works (efficacy). For Rare Disease treatments like Usher syndrome, Phase I and Phase II trials are often combined so they can be done at the same time, making the process move forward faster.
-
Clinical Trial Phase III
The goal of Phase III trials is to show that an investigational drug or treatment approach is safe and effective in a large number of patients.
-
Clinical Trial Phase IV (Treatments)
After FDA approval, treatments are monitored long term for side effects.







