USH1G Research
Gene: SANS
Year Identified: 2003
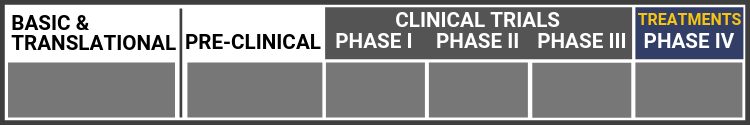
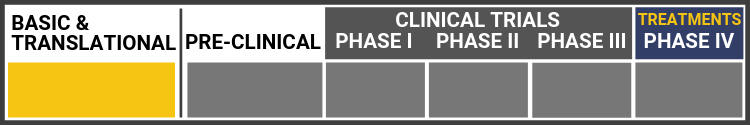
Each research project listed below will include a graphic of the research continuum. The gold box indicates where this project falls on the continuum, illustrating its progress towards reaching people living with Usher syndrome, from "Bench to Bedside."
Click here to learn more about the different stages in the research continuum.
Gene Therapy USH1G


Christine Petit, Ph.D:
Collège de France, Institut Pasteur, Inserm and Sorbonne Université
Petit and her team at Institute Pasteur have partially restored inner ear function and removed almost completely the balance defect in a mouse model of USH1G by viral transfer of the wild-type cDNA to the inner ear of mutant mice shortly after birth. The results provide a basis for future clinical trials in humans.
Dr. Christine Petit's Lab Page
Link to Relevant Publication
Link to Relevant Publication
Elucidating the divers functions of the multipotent scaffold-protein SANS - USH1G


Kerstin Nagel-Wolfrum, Ph.D. and Uwe Wolfrum, Ph.D.:
The USH1G protein SANS (scaffold protein containing ankyrin repeats and SAM domain) is a small scaffold protein. Its numerous interaction partners, identified by “fishing expeditions”, such as yeast-2-hybrids or affinity proteomics tandem, define the different cellular functions of SANS. For further enlightening these functions, the researchers carry on following studies:
a. SANS as part of intracellular transport modules
Their data indicate that SANS is a component of transport carriers, which transport cargoes along microtubule tracks from the trans-Golgi network (TGN) to the base of the cilium in photoreceptor cells. At the ciliary base SANS controls MAGi2-mediated endocytoses and the ciliary import of the microtubule depolymerizing kinesin KIF2A.
b. SANS as a regulator of pre-mRNA splicing
The researchers have shown that SANS regulates splicing of pre-mRNA by interacting with key spliceosome components and controlling the transfer of the tri-snRNP spliceosome subcomplex from the Cajal bodies to the splicing speckles. Defects in USH1G/SANS or its deficiency can lead to defective splicing of target genes. Current research focuses on identifying genes whose mRNA splicing is affected by USH1G/SANS dysfunction. Initial data indicate that other USH and USH-related genes are affected.
c. The role of SANS in the dynamics of membrane-less organelles derived by phase separation
The phenomenon of liquid-liquid phase separation (LLPS) enables the compartmentalization of biopolymers into membrane-less organelles (MLOs). Cellular processes can proceed and be regulated within these compartments by increasing the local concentration of the molecular constituents and modulating their structure and dynamics. In our project we aim to decipher the roles of SANS in the formation of polymer condensates in MLOs at the base of primary cilia and in the nucleus, namely the Cajal bodies and the splicing speckles derived by LLPS.
USH1G-Related Science News
Therapeutic research begins with testing new therapies in laboratory models of the disease before moving to human trials. However, translating success from models (cells or animals) to humans is challenging because they develop function and symptoms at different rates.
Professor Uwe Wolfrum of the Institute of Molecular Physiology at Johannes Gutenberg University Mainz and Professor Reinhard Lührmann at the Max Planck Institute for Biophysical Chemistry in Göttingen have identified a new pathway that leads to Usher syndrome (USH).
This 2010 review dives into the genetics of pathological mechanisms of Usher syndrome.
What this means for Usher syndrome: Research has come a long way since 2010!